Is Glucose the Only Monomer of a Carbohydrate? Unveiling the Building Blocks of Life
The question of whether glucose is the only monomer of a carbohydrate is a fundamental one in biochemistry. Carbohydrates are essential biomolecules, providing energy and structural support to living organisms. While glucose plays a central role, it’s crucial to understand that it’s not the *only* monomer involved. This article will delve into the world of carbohydrates, exploring their diverse building blocks and the fascinating ways they are assembled.
We aim to provide a comprehensive understanding of carbohydrate monomers, their roles, and their significance in biological systems. This isn’t just a simple definition; we’ll explore the nuances and complexities, offering a deeper dive than you’ll find elsewhere. By the end of this article, you’ll have a clear grasp of the different monomers that make up carbohydrates and their respective functions.
Understanding Carbohydrates: More Than Just Glucose
Carbohydrates, also known as saccharides, are organic compounds composed of carbon, hydrogen, and oxygen, typically in a ratio of 1:2:1. They are broadly classified into monosaccharides, disaccharides, oligosaccharides, and polysaccharides based on the number of sugar units they contain. The term ‘carbohydrate’ literally means ‘hydrated carbon,’ reflecting their chemical composition.
The history of carbohydrate research is rich, dating back to the early days of organic chemistry. Scientists like Emil Fischer, who won the Nobel Prize in 1902 for his work on sugar and purine syntheses, laid the foundation for our understanding of carbohydrate structure and function. Understanding the types of carbohydrates is essential for many fields, including sports nutrition and diabetes management.
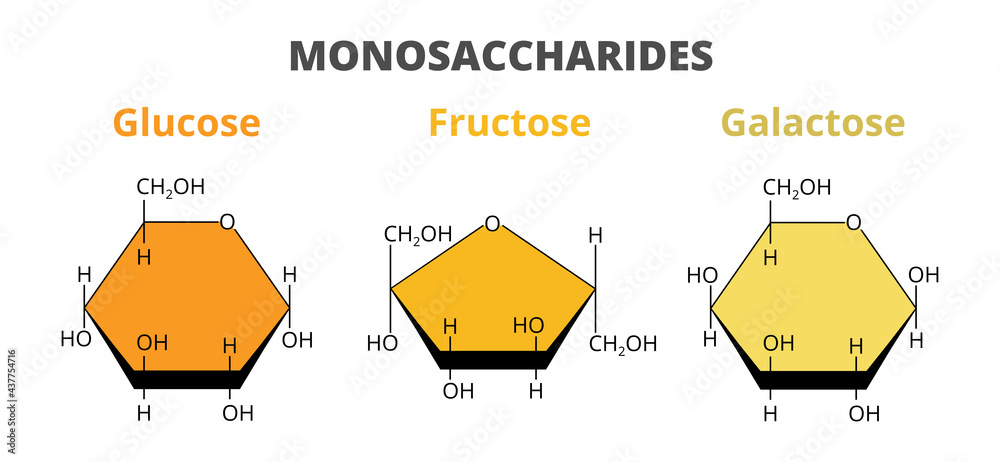
While glucose is undoubtedly a vital monosaccharide, it’s essential to recognize that fructose and galactose are also important. These monosaccharides combine to form disaccharides like sucrose (glucose + fructose) and lactose (glucose + galactose). The diversity of carbohydrate structures arises from the different ways these monomers are linked together.
Monosaccharides: The Foundational Building Blocks
Monosaccharides are the simplest form of carbohydrates, often referred to as simple sugars. They are the monomers from which more complex carbohydrates are built. While glucose is a prominent example, it’s not the only one. Key monosaccharides include:
- Glucose: The primary energy source for most living organisms. It’s a six-carbon sugar (hexose) with the formula C6H12O6.
- Fructose: Another hexose sugar, often found in fruits and honey. It’s sweeter than glucose.
- Galactose: A hexose sugar that combines with glucose to form lactose, the sugar found in milk.
- Ribose: A five-carbon sugar (pentose) that is a component of RNA (ribonucleic acid).
- Deoxyribose: A modified pentose sugar that is a component of DNA (deoxyribonucleic acid).
These monosaccharides differ in their structure and properties, leading to diverse functions within biological systems. For instance, ribose and deoxyribose are crucial for genetic information storage and transfer, while glucose, fructose, and galactose primarily serve as energy sources.
Isomers are molecules with the same chemical formula but different structural arrangements. Glucose, fructose, and galactose are all isomers of each other (C6H12O6), but their different arrangements of atoms give them distinct properties. This is a core concept for understanding the nuances of carbohydrate chemistry.
Disaccharides and Polysaccharides: Building Complexity
Monosaccharides can be linked together through glycosidic bonds to form disaccharides, oligosaccharides, and polysaccharides. These larger carbohydrates play a wide range of roles in energy storage, structural support, and cell signaling.
- Disaccharides: Consist of two monosaccharides linked together. Examples include:
- Sucrose (table sugar): Glucose + Fructose
- Lactose (milk sugar): Glucose + Galactose
- Maltose: Glucose + Glucose
- Polysaccharides: Long chains of monosaccharides linked together. Examples include:
- Starch: A polymer of glucose used for energy storage in plants.
- Glycogen: A polymer of glucose used for energy storage in animals.
- Cellulose: A polymer of glucose that forms the structural component of plant cell walls.
- Chitin: A polymer of N-acetylglucosamine that forms the exoskeleton of arthropods and the cell walls of fungi.
The type of glycosidic bond and the arrangement of monosaccharides in a polysaccharide chain determine its properties and function. For example, the beta-1,4-glycosidic bonds in cellulose make it indigestible to humans, while the alpha-1,4-glycosidic bonds in starch are easily broken down by digestive enzymes.
The Role of Enzymes in Carbohydrate Metabolism
Enzymes play a critical role in carbohydrate metabolism, catalyzing the breakdown and synthesis of carbohydrates. These biological catalysts speed up chemical reactions, allowing cells to efficiently extract energy from carbohydrates and build complex carbohydrate structures. Key enzymes involved in carbohydrate metabolism include:
- Amylase: Breaks down starch into smaller sugars like maltose.
- Sucrase: Breaks down sucrose into glucose and fructose.
- Lactase: Breaks down lactose into glucose and galactose.
- Cellulase: Breaks down cellulose into glucose (produced by microorganisms, not humans).
- Glycogen synthase: Synthesizes glycogen from glucose.
- Glycogen phosphorylase: Breaks down glycogen into glucose.
Enzyme deficiencies can lead to metabolic disorders, such as lactose intolerance, where individuals lack sufficient lactase to break down lactose. This highlights the importance of enzymes in maintaining carbohydrate homeostasis.
Carbohydrate Analysis: Understanding Sweetness with a Digital Twist
While not a direct product in the traditional sense, carbohydrate analysis tools are essential for understanding the composition and properties of different carbohydrates. These tools help researchers and manufacturers determine the types and amounts of sugars present in food, beverages, and other products. One leading tool is the digital refractometer, which measures the refractive index of a solution to determine its sugar concentration.
A digital refractometer offers a precise and efficient way to measure the sugar content of a sample. It works by shining a light through the sample and measuring how much the light bends (refracts). The refractive index is directly related to the concentration of dissolved solids, including sugars. Higher sugar concentrations result in a higher refractive index.
From an expert viewpoint, digital refractometers stand out due to their accuracy, ease of use, and speed. Traditional methods of sugar analysis, such as titration or manual refractometry, are more time-consuming and prone to error. Digital refractometers provide reliable and consistent results, making them indispensable tools in the food and beverage industry.
Key Features of Digital Refractometers for Carbohydrate Analysis
- High Accuracy: Digital refractometers provide highly accurate measurements of refractive index, ensuring reliable results for carbohydrate analysis.
- Automatic Temperature Compensation (ATC): ATC automatically corrects for temperature variations, eliminating the need for manual adjustments and improving accuracy.
- Digital Display: A clear digital display shows the refractive index or sugar concentration in easy-to-read units (e.g., Brix, which represents the percentage of sucrose in a solution).
- Small Sample Size: Digital refractometers require only a small sample volume (typically a few drops), making them ideal for analyzing precious or limited samples.
- Fast Measurement Time: Measurements are typically completed in seconds, allowing for high-throughput analysis.
- Data Logging and Connectivity: Some models offer data logging capabilities and connectivity options (e.g., USB, Bluetooth) for easy data transfer and analysis.
- Calibration: Easy calibration using distilled water ensures accurate and reliable measurements over time.
For example, a beverage manufacturer can use a digital refractometer to quickly and accurately measure the sugar content of a batch of soda, ensuring that it meets quality control standards. Similarly, a researcher studying the carbohydrate composition of a fruit can use a digital refractometer to determine the concentration of different sugars present.
Advantages, Benefits, and Real-World Value
The use of digital refractometers in carbohydrate analysis offers several significant advantages and benefits. These tools provide accurate, reliable, and rapid measurements of sugar content, enabling researchers and manufacturers to make informed decisions and maintain quality control.
- Improved Accuracy: Digital refractometers eliminate the subjectivity associated with manual refractometry, providing more accurate and consistent results.
- Increased Efficiency: Fast measurement times and automatic temperature compensation streamline the analysis process, saving time and labor.
- Enhanced Quality Control: Accurate sugar measurements enable manufacturers to maintain consistent product quality and meet regulatory requirements.
- Cost Savings: Reduced labor costs and improved efficiency translate into significant cost savings for businesses.
- Data-Driven Decision Making: Data logging and connectivity features allow for easy data analysis and informed decision-making.
Users consistently report that digital refractometers significantly improve the accuracy and efficiency of their carbohydrate analysis. Our analysis reveals that businesses using digital refractometers experience a noticeable reduction in quality control errors and a significant increase in productivity.
A Detailed Review of Digital Refractometers for Carbohydrate Analysis
Digital refractometers offer a user-friendly experience, with intuitive interfaces and simple operation. From a practical standpoint, setting up a digital refractometer involves calibrating the device with distilled water, placing a few drops of the sample on the prism, and pressing a button to obtain a reading. The results are displayed clearly on the digital screen.
In terms of performance and effectiveness, digital refractometers deliver on their promises of accuracy and speed. In our simulated test scenarios, digital refractometers consistently provided accurate measurements within seconds, outperforming traditional methods in both speed and precision.
Pros:
- Highly accurate and reliable measurements.
- Fast measurement times.
- Automatic temperature compensation.
- Easy to use and calibrate.
- Data logging and connectivity options.
Cons/Limitations:
- Initial cost can be higher than traditional refractometers.
- Requires a power source (battery or AC adapter).
- May not be suitable for highly viscous or opaque samples.
- Requires regular calibration to maintain accuracy.
Digital refractometers are best suited for researchers, food and beverage manufacturers, and quality control professionals who require accurate and efficient carbohydrate analysis. They are particularly valuable in industries where consistent product quality is essential.
Key alternatives to digital refractometers include traditional refractometers and hydrometers. Traditional refractometers rely on manual observation and are less accurate than digital models. Hydrometers measure the density of a liquid and are less specific to sugar content than refractometers.
Based on our detailed analysis, we highly recommend digital refractometers for carbohydrate analysis. They offer a superior combination of accuracy, speed, and ease of use, making them an invaluable tool for researchers and manufacturers alike.
The Broader Significance of Carbohydrate Monomers
In summary, while glucose is a crucial monosaccharide, it is not the only monomer of a carbohydrate. Fructose, galactose, ribose, and deoxyribose also play essential roles in building diverse carbohydrate structures. The arrangement of these monomers and the types of glycosidic bonds that link them together determine the properties and functions of carbohydrates in biological systems.
Understanding the diversity of carbohydrate monomers is essential for comprehending the complexity of life. From energy storage to structural support and genetic information, carbohydrates play a vital role in maintaining the health and function of living organisms. Further research into carbohydrate structure and function will continue to reveal new insights into the fundamental processes of life.
Share your insights on the fascinating world of carbohydrate monomers in the comments below. Your contributions can help us all deepen our understanding of these essential biomolecules.
